The vibrational degrees of freedom for a diatomic molecule are dead at
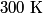 (room temperature), while the rotational degrees of freedom are active at (room temperature), while the rotational degrees of freedom are active at 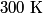 . When the temperature is increased beyond . When the temperature is increased beyond 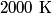 , the vibrational degrees of freedom become active for , the vibrational degrees of freedom become active for 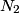 (nitrogen) and (nitrogen) and 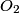 (oxygen). Thus, answer (E) is correct. (oxygen). Thus, answer (E) is correct. |